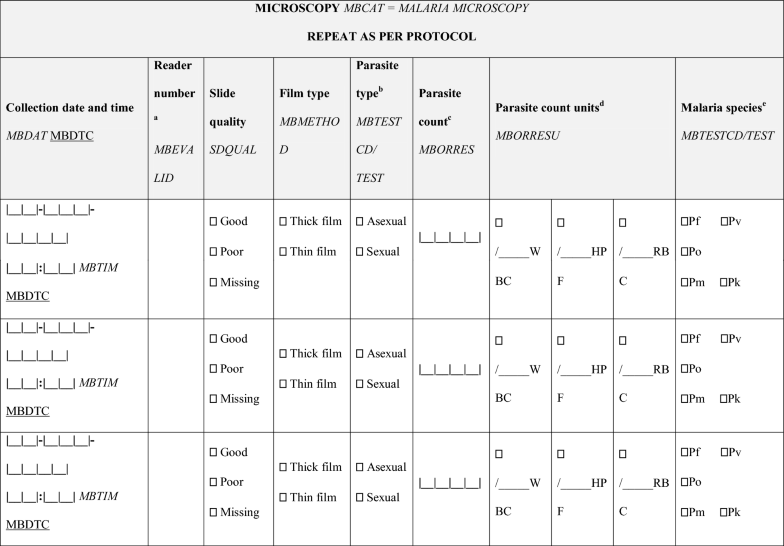
- Clinical Data Acquisition Standards Harmonization (CDASH) annotations are in italics; Standard Data Tabulation Module (SDTM) annotations are underlined
- aFor studies that require the slide to be read by more than one microscopist, include a separate row for the results from each reader
- bComplete a separate row for asexual and sexual parasites if seen on the same slide. Adapt if additional information on the staging of asexual parasites may be required by the study protocol; e.g. rings, trophozoites, schizonts
- cRecord the actual parasite count per WBC/HPF/RBC; conversion into parasites per µL are performed separately
- dThe preferred method of calculating parasite density uses actual WBC/μL; some study protocols may assume (xxx) WBC/μL or use the ‘HPF’ method
- eIn cases of mixed infections, all infecting species must be reported; however, the asexual and/or sexual parasite count need not be reported separately for each species unless specifically required in the study protocol. If species are reported separately, counts for each species must be entered on separate lines. Asexual and sexual stages from the same slide/parasite species also must be entered on separate lines

