- Review
- Open access
- Published:
The behaviour of adult Anopheles gambiae, sub-Saharan Africa’s principal malaria vector, and its relevance to malaria control: a review
Malaria Journal volume 23, Article number: 161 (2024)
Abstract
Background
Mosquitoes of the Anopheles gambiae complex are one of the major vectors of malaria in sub-Saharan Africa. Their ability to transmit this disease of major public health importance is dependent on their abundance, biting behaviour, susceptibility and their ability to survive long enough to transmit malaria parasites. A deeper understanding of this behaviour can be exploited for improving vector surveillance and malaria control.
Findings
Adult mosquitoes emerge from aquatic habitats at dusk. After a 24 h teneral period, in which the cuticle hardens and the adult matures, they may disperse at random and search upwind for a mate or to feed. Mating generally takes place at dusk in swarms that form over species-specific ‘markers’. Well-nourished females may mate before blood-feeding, but the reverse is true for poorly-nourished insects. Females are monogamous and only mate once whilst males, that only feed on nectar, swarm nightly and can potentially mate up to four times. Females are able to locate hosts by following their carbon dioxide and odour gradients. When in close proximity to the host, visual cues, temperature and relative humidity are also used. Most blood-feeding occurs at night, indoors, with mosquitoes entering houses mainly through gaps between the roof and the walls. With the exception of the first feed, females are gonotrophically concordant and a blood meal gives rise to a complete egg batch. Egg development takes two or three days depending on temperature. Gravid females leave their resting sites at dusk. They are attracted by water gradients and volatile chemicals that provide a suitable aquatic habitat in which to lay their eggs.
Conclusion
Whilst traditional interventions, using insecticides, target mosquitoes indoors, additional protection can be achieved using spatial repellents outdoors, attractant traps or house modifications to prevent mosquito entry. Future research on the variability of species-specific behaviour, movement of mosquitoes across the landscape, the importance of light and vision, reproductive barriers to gene flow, male mosquito behaviour and evolutionary changes in mosquito behaviour could lead to an improvement in malaria surveillance and better methods of control reducing the current over-reliance on the indoor application of insecticides.
Background
Members of the Anopheles gambiae complex of mosquitoes have probably been responsible for more human deaths than any other animal (see Appendix), principally because they are exceptionally efficient transmitters of Plasmodium falciparum, the most lethal form of malaria [1, 2]. This mosquito complex is a highly efficient vector of disease for four main reasons: (1) they are often highly abundant, (2) frequently bite people, (3) are highly susceptible to infection and (4) are long-lived (for a mosquito) [3]. Factors such as their propensity to enter houses to feed, the host preference and behaviour after feeding are also important. Anopheles gambiae sensu stricto (s.s.), Anopheles coluzzii and Anopheles arabiensis are the three members of the complex that, with Anopheles funestus, are the primary vectors of malaria in sub-Saharan Africa.
Whilst the malaria parasite has adapted to being transmitted by these mosquitoes, the insects have adapted their ecology and behaviour to exploit humans. People unwittingly create aquatic habitats for the immature stages of the mosquito around their homes and in nearby fields, and the adult female mosquito enters houses and feeds on people at night when they are least able to defend themselves. This review describes the journey of a female An. gambiae from its emergence to locating and feeding on a human host, before eventually laying its eggs. It illustrates how a deeper understanding of these behaviours might lead to the development of novel methods of vector surveillance and control. The review is primarily intended as an introduction to the behaviour of this important vector for students and early career scientists updating earlier reviews on the subject [4,5,6,7,8].
The Anopheles gambiae complex
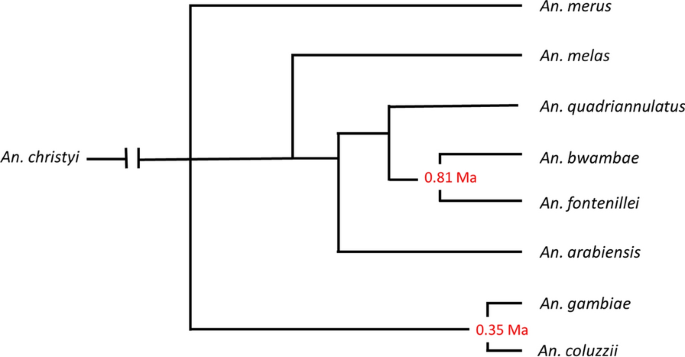
The Anopheles gambiae species complex consists of at least eight morphologically indistinguishable species, most of which are primarily zoophilic. Two of the most closely related members of the complex, An. gambiae and An. coluzzii, are primary malaria vectors due to their tendency to feed on humans, being long-lived, due to their resting inside traditional thatch roofed houses, and their relatively high abundance [9,10,11]. Anopheles gambiae occurs throughout much of sub-Saharan Africa whilst An. coluzzii is limited in its distribution to West Africa [12]. They are the most recently diverged members of the complex and share many behavioural traits (Fig. 1). Anopheles coluzzii was newly reported from East Africa for the first time suggesting that members of the complex are extending their range [13].
Speciation in the An. gambiae complex (source: Barron et al. [10]). Note that Anopheles amharicus, closely related to An. quadriannulatus, is not yet included in this figure. Numbers represent the divergence (Ma, million years ago) estimated based on the pairwise distances of the ML phylogeny and assuming a substation rate of 11 × 10–9 per site, per generation and 10 generations per year [38]
Until very recently humans improved the niche occupied by malaria vectors. In addition to providing a suitable larval habitat, humans provided a suitable host and so anthropophily (when blood-feeding insects prefer to feed on human blood) developed. As a side effect of anthropophily, as people improved their housing, endophily, the habit of resting inside, evolved [14]. It was these two behaviours (anthropophily and endophily) that made An. gambiae an exceptional vector of malaria. As pointed out by Gillies and Coetzee [15] species isolation between members of the complex ‘presumably involves a spatial element’. Spatially separated swarms, where mating takes place, is the mate-recognition system that maintains isolation between members of the An. gambiae complex. It is the aspect of mating behaviour that has been most thoroughly investigated, especially by Diabate and colleagues in West Africa, reviewed in Sawadago et al. [16].
Anopheles arabiensis is also a primary malaria vector in some circumstances. It has opportunistic feeding habits and will feed on both human and animal hosts, can reach high population densities but is less long lived than the other two vectors due, perhaps, to a tendency to rest outdoors [17]. It is also more drought resistant, perhaps because of its larger size, than the other two vectors and has a greater geographical distribution [18]. Due to its tendency to both feed and rest outdoors it is less susceptible to control measures, such as insecticide treated nets or indoor resudual spraying, aimed at insects which feed or rest inside houses. In a number of instances, it has replaced An. gambiae as the most common member of the complex when such control measures have been introduced [17, 19, 20].
Other members of the complex (Anopheles bwambae, Anopheles merus, Anopheles melas, Anopheles amharicus and Anopheles quadriannulatus and, the recently described, Anopheles fontenillei) since they are limited in their distribution and are largely zoophilic, are only ever of minor importance as vectors.
Life cycle of Anopheles gambiae
As with all mosquitoes, An. gambiae exploits three habitats: an aquatic environment for early development, an aerial environment where host-seeking and mating takes place and a terrestrial environment where feeding and egg production take place. Eggs are laid singly, hatch within two to three days and pass through four larval stages in seven to 10 days, depending on water temperature, food availability and quality [21, 22]. Larvae feed on organic material in the water, gently moving food into their mouths using bristles. This food is used for metabolic energy and build-up of energy reserves needed to survive the first few days as an adult mosquito [23]. Larval development, which is the only time when growth occurs, is followed by pupation that lasts two to three days [24]. In the laboratory, the period from laying to adult emergence takes 10–23 days [24, 25]. In a natural environment, daily temperature variations affect the duration of the mosquitoes’ development. Perhaps because of their slighter build, males emerge a day or two before females.
Behaviours of Anopheles gambiae
Emergence and dispersal
Adult anophelines emerge from the pupae soon after dusk [26] and wait several minutes before inflating their wings and drying out, before flying off. The dispersal flight varies according to wind strength and direction. If there is little or no wind, dispersal can be random, but if there is a strong predominant wind, mosquitoes may be blown with the wind [27]. In open savanna, 80% of An. gambiae fly less than one metre above the ground [28, 29] with a maximum flight speed of 1.4–1.8 ms−1 [30]. Flight occurs at night and is under control of circadian rhythms [31]. Studies using flight actographs measuring the activity of individual mosquitoes show a primary peak after sunset, corresponding to the period for dispersal and oviposition, followed by secondary activity with a peak after midnight, corresponding to host location behaviour (Fig. 2).
Estimations of the distance Anopheles mosquitoes disperse is challenging given the heterogeneity in techniques used and the large variation in the ecology of sites [32]. Dispersal depends not only on the proximity, size and abundance of aquatic habitat to human habitation, but also how humans and alternative sources of blood meal are distributed in the landscape, the local vegetation and abiotic environmental conditions (incl. wind, relative humidity). The relative size and proximity of both aquatic habitats and human habitation is key. In the central part of The Gambia, where the river floods during the rainy season producing extensive pooling, An. melas may fly over 2 km from aquatic habitats to villages that are spatially clustered [33]. Support for this comes from the lack of an effect on indoor mosquito densities where large-scale larviciding was done in a 2 km area surrounding the study villages [34]. In marked contrast, in a village in western Kenya, aquatic habitats were largely human made and highly clustered within a village where houses were more widely dispersed [35, 36], suggesting short flight distances after emergence to blood feeding. When the wind is strong (> 1.2 ms−1), as occurs immediately preceding a tropical storm, there may be little flight activity. Passive transportation of a few individuals moving with storm fronts at high altitude has been observed [37, 38]. Whether mosquitoes that are lifted up and transported over long distances in this way survive their journey is open for debate. Even short suspension in cages at altitude reduced survival considerably [39]. Mosquitoes may also be transported long distances by planes, ships, trains and vehicles [5]. The introduction of An. arabiensis [40] into Brazil probably occurred by the fast mailboats from West Africa, whilst spread by vehicles was considered so important that fumigation posts were established to prevent the spread of adult mosquitoes [41].
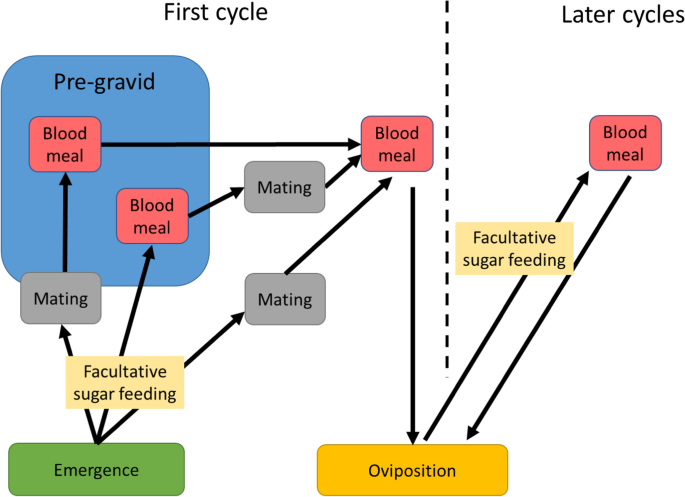
After emergence, the behaviour of females of the complex is associated with three phases of adult life: mating, blood feeding and oviposition (Fig. 3). Some individuals imbibe sugar as well (see below). Newly emerged An. gambiae complex females may exhibit “pre-gravid” behaviour in which a proportion of the females require two blood meals before they can complete egg maturation [42, 43]. It has been suggested that the first blood meal is required to build up metabolic energy reserves when females emerge undernourished from the pupa [44] These females usually also have a smaller body size compared to their well-fed siblings and may feed before mating [43].
Behaviours of adult mosquitoes. Note that sugar feeding is facultative. Under natural conditions, variable proportions of recently emerged An. gambiae complex females may undergo a pre-gravid phase before completing their gonotrophic cycle. i.e. the period between one egg laying and the next. These females are often undernourished and require two bloodmeals in order to complete their first gonotrophic cycle [43]. A first bloodmeal may be necessary before mate-seeking behaviour takes place [44]. In the pre-gravid state (inside the blue box) females do not sugar feed as they derive sufficient nutriments from the first blood meal for mate location
Sugar feeding
Newly-emerged adults fly to a resting site on nearby plants, such as grasses and shrubs, where they may remain until searching for a sugar meal guided by odorant volatiles from plants to which these mosquitoes are attracted [45,46,47]. Sugar provides a source of readily available energy whilst blood, necessary for the production of eggs, can also be used for this purpose but less efficiently [44, 48]. Sugar may be taken from honeydew of extra-floral nectaries of plants like Mangifera indica (mango), Dolonix regia (flamboyant or rural poinciana), Thevetia neriifolia (yellow oleanda), Cassia siberiana (drumstick tree), Parthenium hysterophorus (Santa Maria feverfew) and others [45, 49]. These sugars provide metabolic energy required for flight and mating. After a single sugar meal, females generally switch to mating followed by host-seeking and blood feeding [50]. As in all biological systems, there occurs some variation in behaviour, and recent evidence suggests that female anophelines may imbibe a sugar meal, after blood feeding [51, 52] due to the physiological condition of the female. Males continue to feed on sugar as their only source of nutrition.
Mating behaviour
As in other mosquito species, newly emerged male An. gambiae need to mature sexually before mating. During this process, their genitalia rotate 180° within a day or at most two days [53]. Females become receptive to males following a nectar feed [54, 55], or an initial blood meal, with mating taking place in swarms [55,56,57,58,59].
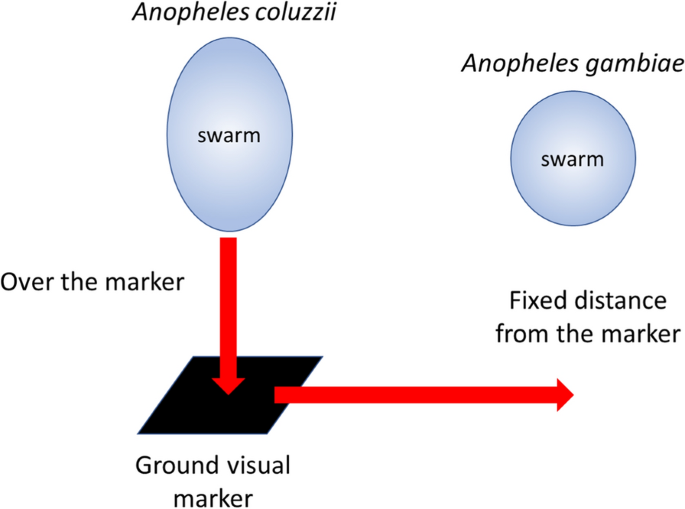
Swarms are characterized by the males flying in a stationary holding pattern over, or in relation to, some aspect of the environment that is species specific. Thus, An. coluzzii swarms directly over horizontal areas of contrast [59] whilst An. gambiae tends to swarm to the side of the same markers [60] ensuring some measure of spatial separation (Fig. 4). This behaviour limits hybridization in areas where the two species are sympatric. Although an individual mosquito can swarm, swarms generally contain anything from tens to 100’s of insects. The size of any swarm depends to a certain extent on the mosquito population density and the size and attractiveness of the marker [61, 62]. Virgin females respond to the same visual cues as males and in the absence of males will themselves undertake short swarming flights [56, 60]. If a swarm is present, then the female will be mated.
Swarming behaviour of An. gambiae. Note that swarms of An. gambiae are more spherical than An. coluzzii (after [60])
Swarms form for a limited time, circa 30 min, at dusk [16]. Males are attracted to the flight tone of the female which they hear using their antennae. Males beat their wings around 600 times a second (600 Hz), whereas females beat their wings at around 440 Hz [63]. Thus, the flight tones of males and females differ and males do not respond to the flight tone of other males but only to that of the female. They only hear the female when the fibrillae on their antennae are erect, which happens shortly before flight activity and which is under the control of a circadian clock [64]. As a result, receptivity to the female flight sound is limited to a relatively short period [56, 65].
Swarms can sometimes involve hundred and even thousands of individuals, hence their dynamics is particularly intriguing. It has been recently shown that the dynamics of these and other animal aggregations such as of birds, fish and insects can be recreated using relatively simple mechanistic computer models that can simulate flocking in birds, shoaling in fish and swarming in insects (Langton 1996, quoted in [66]). Thus, males and females, only need to: 1) move towards the perceived centre of mass of the insects in its neighbourhood; 2) match velocities with insects in its neighbourhood; and 3) maintain a minimum distance from other objects in the environment, including other mosquitoes. When a female enters a swarm males will follow rule #2 and match speeds (and therefore their flight tone) with the female [67, 68]. Swarm size appears not to affect mating success and it is not clear even if females select a mate [69, 70].The timing of mating is fixed throughout the year and occurs shortly before sunset. Between two closely related species, An. gambiae and An. coluzzii, there is small difference in onset of mating, which helps to keep the reproductive isolation of the two species [16].
Mating has also been reported to occur inside houses but what distinguishes this from the usual process is not known [71, 72]. Males can mate multiple times throughout adult life, but given the overall equal sex ratio, and female monogamy, they are unlikely to do so.
During mating, substances from the male accessory glands will form a mating plug inside the bursa copulatrix, which effectively blocks off the entrance to the spermatheca [73, 74]. This substance contains the steroid hormone 20-hydroxyecdysone, which induces mating-refractory behaviour in the female and stimulates oviposition [75].
Host location
On approaching a host, female mosquitoes may track upwind, turning into the odour stream and moving up the concentration gradient. Field experiments indicate that mosquitoes can locate host outdoors, principally carbon dioxide, from a distance of 35 m [76].
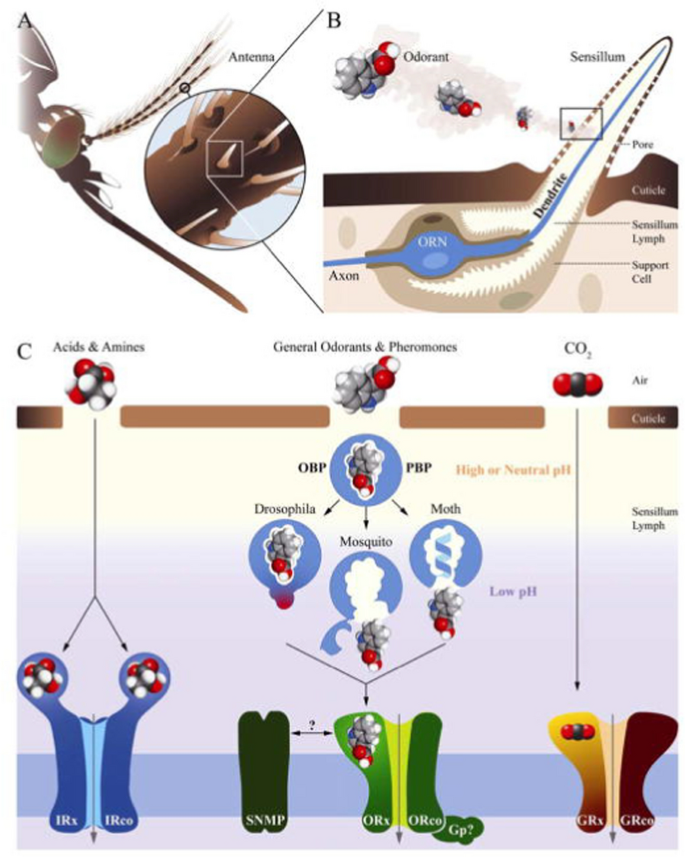
Hosts are recognized by carbon dioxide and olfactory cues (body odours mostly), emanating from the human skin. The major attracter of longer distances is carbon dioxide [76], which is a general indicator of a mammal, but closer to the host a female will be activated by a range of volatile organic compounds (VOCs) that are produced by a multitude of microbial organisms [77, 78]. Human odours include ammonia, l-lactic acid, tetradecanoic acid, 3-methyl-1-butanol and butan-1-amine [77, 79, 80]. Artificial baits, or lures, made from these VOCs, together with carbon dioxide, provide excellent ways to attract mosquitoes. These odorant cues are being recognized by selected neural receptors located on the antennae (Box 1). When stimulated by an odourant molecule, a signal will be passed through the neurons to the mosquito brain, inducing a behavioural response [81,82,83].
a An insect chemosensory system and molecular models in signal transduction. b An olfactory sensillum housing support cells and an olfactory neuron (blue). c Various groups of chemoreceptors being activated by different classes of odorants. Legend modified after [83]
Although underexplored, vision is also important for the orientation of mosquitoes. Based on studies on night-flying mosquitoes in the 1970s and 1980s [84,85,86,87,88,89], scientists showed that mosquitoes were attracted to visually conspicuous objects at a distance, although they avoided solid objects at close range. Although none of these studies involved An. gambiae, Gillies and Wilkes suggested that house-entering mosquitoes could be attracted to the shape of the house over 15–20 m, particularly if it was isolated from other houses or tall vegetation. Indoor lighting, visible from outside, can also attract An. gambiae into an experimental hut [89]. In this experiment, 84% more mosquitoes were collected in light-traps in huts with transparent walls than those with opaque walls [90]. The range of attraction of light is likely to be in the region of 5 m [90, 91], and may vary with wavelength and intensity of light. In marked contrast, light added to the Furvela tent-trap resulted in a reduced catch compared to traps without light [92] and evidence suggests that when used outdoors CDC light-traps, whilst operationally practical, do not adequately sample the outdoor biting fraction of malaria vectors [93].
As mosquitoes approach an unprotected host, temperature and relative humidity also act as attractants increasing biting rates [94, 95]. Feeding on a sitting host is primarily concentrated around the ankles and feet of individuals [96, 97].
House entry
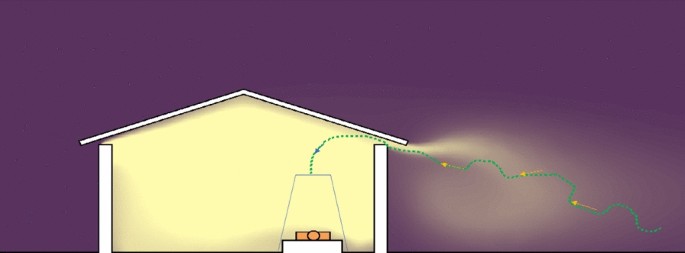
The presence of a gap between the top of the wall and the roof, or at the gable ends of a house are common features of traditional thatched-roof African houses (Fig. 5). It is through these gaps that host-seeking mosquitoes will enter [98, 99]. Recent studies show that the relative attractiveness of a house increases if there is a high concentration of carbon dioxide emanating from inside [102, 103]. As might be expected, the density of mosquitoes entering an occupied house increases with an increasing number of residents [102, 103]. Increasing ventilation, which dilutes the carbon dioxide, will have the opposite effect reducing house entry by An. gambiae [100, 101].
How Anopheles gambiae enters a house through the open eaves. Yellow represents human odours and the dashed line the trajectory of a blood-questing mosquito (After Spitzen et al. [104]). The human host is protected by a bed net
As a female An. gambiae approaches within several metres from a house, she will gain altitude and enter the building through the open eaves [104]. Raising a house on stilts several metres above the ground will reduce house entry of An. gambiae, probably due to reduced levels of human odour at ground level and lack of a wall to aid elevated flight [105]. In The Gambia, an experimental hut three metres above the ground housing two men had 84% fewer An. gambiae than a similar house on the ground. Nonetheless, adding netting or solid walls to an elevated hut will reduce this effect, increasing the numbers of mosquitoes entering the elevated room [106]. Similarly, in São Tomé, people living in houses built off the ground have significantly fewer An. coluzzii attacking them than people in houses at ground level [107].
Once inside the bedroom, an An. gambiae female will be attracted to hosts through volatiles produced by the occupants. Video recording shows that 75% of mosquitoes approach a human-occupied insecticide-treated bed net (ITN) from the top of the net around the torso, with a few individuals landing on the sides [108]. The behaviour of the mosquitoes was categorized into swooping, visiting, bouncing and resting. This behaviour is likely to be due to the warm odours rising from the host rising within the net as indicated using computational fluid dynamic modelling [109].
Variation in attractiveness to mosquitoes
Adult humans differ markedly in their relative attractiveness to An. gambiae, with some individuals receiving substantially more bites than others [110,111,112] with evidence that this variation is partly under genetic control [113]. Because of their smaller size, children are less attractive to mosquitoes than adults [110, 114], whilst pregnant women are more attractive to mosquitoes because of their larger size and more active metabolism compared to their non-pregnant sisters [115, 116]. Variation in attractiveness is partly explained by the variation in the skin microbiome, which results in differences in VOCs [78, 117, 118] Odours from human skin may be repellent or attractive [78], and thus provide an opportunity for selection of cues that could be used for prevention against mosquito bites. People with malaria are more attractive to mosquitoes, although this relationship is not clear. People infected with gametocytes appear to be more attractive to An. gambiae than uninfected people [119,120,121]. The increase in attractiveness was associated with raised concentrations of aldehydes.
After An. gambiae has identified a potential host, the combination of odours, skin humidity and body temperature induce a landing response followed by biting. Recent studies showed that odour, body heat and visual stimuli act synergistically in causing a landing response in An. gambiae mosquitoes [122]. In field studies in Burkina Faso, these stimuli combined attracted significantly more An. gambiae than either stimulus alone. Indeed, a thermal stimulus was required to obtain an optimal result [95, 123]. In Malawi, An. arabiensis was similarly attracted to a warm human decoy [123].
Blood feeding behaviour
When a suitable site for biting has been found, blood feeding can begin, taking up to 3 min to complete. Detailed accounts of the feeding behaviour of mosquitoes include Gordon and Lumsden [124], Robinson [125], Griffiths and Gordon [126], Christophers [127] and Ribeiro [128].
The labrum plays a central role in blood uptake, as it encloses the food channel [129]. Sensory receptor cells located at the tip of the labrum detect blood quality and even direct the labrum towards a blood vessel [130]. Once blood has been detected, on average, 3 µl of blood is ingested. During feeding, diuresis of the watery parts of blood occurs, considerably reducing excess bodyweight from the blood meal and allowing the mosquito to take flight and search for a resting site. It also allows the mosquito to cool off [131].
Mosquitoes with malaria parasite in their salivary glands will probe more frequently than uninfected mosquitoes [132, 133]. This behaviour results from destruction of parts of the salivary gland, reducing apyrase production, an important enzyme that prevents blood clotting. This behaviour has been observed with malaria-infective An. gambiae [134] and would partly explain multiple cases of malaria in the same house at the same time [135, 136].
Diel biting patterns
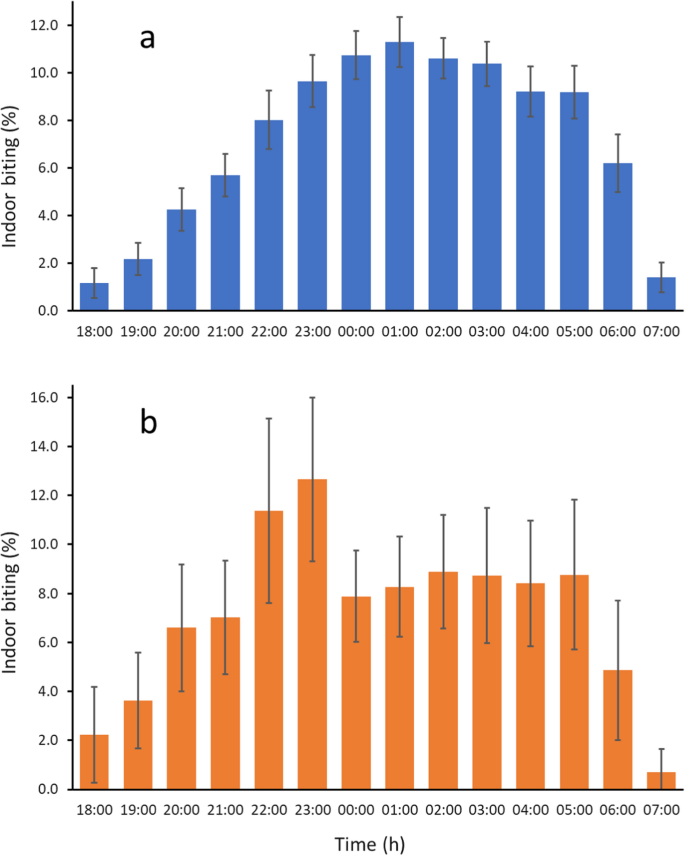
The circadian activity rhythms (Fig. 2), distance and abundance of aquatic habitats and human population, topography, vegetation, housing quality and weather can affect the shape of the biting cycles of An. gambiae. This probably explains why biting patterns can vary markedly from site to site [5, 137]. Yet, summarizing 92 recent studies from sub-Saharan Africa [138] showed that there was a clear tendency for An. gambiae biting activity to increase from dusk to a maximum at 01.00 h before slowly declining until 05.00 h, before dropping precipitously to low levels at 07.00 h (Fig. 6).
Diel biting activity of a, An. gambiae s.l. and b, An. arabiensis. Error bars are 95% confidence intervals. Data based on 92 and 17 data sets respectively. Data from Sherrard-Smith et al. [137] (data collected from 1978 to 2017)
This analysis also showed that An. arabiensis tends to bite earlier in the night than An. gambiae and An. coluzzii (Fig. 6b). Indeed in some cases there is marked outdoor biting early in the evening [138, 139]. In Sierra Leone young (nulliparous) An. gambiae bit earlier than parous ones [140]. In general, however, parous and nulliparous biting cycles are similar [5]. Mating does not appear to influence host-seeking behaviour in the An. coluzzii from the archipelago of São Tomé and Príncipe [141].
The paradigm of night feeding of An. gambiae (both An gambiae s.s. and An. coluzzii) may need to be revised, however, following the results of a recent study in the Central Africa Republic [142]. It is reported that in this study 10 to 30% of indoor bites occurs during daytime. However, this activity need not necessarily be linked to the insects circadian activity (and so is a facultative behaviour, rather than an intrinsic one). This study demonstrates that studies on An. gambiae feeding behaviour should be expanded to other regions to explore the nature of these behavioural changes.
Behavioural differences
There is a tendency to provide labels of behaviour which appear fixed, such as when mosquitoes feed on animals (zoophagy) or human hosts (anthropophagy), feed inside (endophagy) or outside houses (exophagy) or rest inside (endophily) or outside houses (exophily). This is often not the case, An. gambiae can be highly variable in its behaviour throughout its range. For example, if houses are well built, with few if any holes, An. coluzzii, that might otherwise feed on humans indoors, will feed and rest outside, on whatever hosts are available, as is the case on the archipelago of São Tomé and Principe. As pointed out by Levèvre et al. [143] ‘the highly anthropophilic label given to An. gambiae s.s. must be carefully interpreted and refer to populations rather than the whole sibling species. The same is true for endophagy and exophagy. Thus, although often considered to bite predominantly outdoors, one in three blood meals among An. arabiensis from Tanzania took place indoors [145]. Similarly, in a study site from Ethiopia, where people slept outdoors next to their cattle, 46% of resting An. arabiensis collected outdoors had fed on humans despite the high cattle: human ratio (17:1) [145]. Such results suggest that the An. arabiensis population was inherently anthropophagic, although this was counterbalanced by exophagic and exophilic tendencies in the mosquito [146]. Although in An. gambiae and An. coluzzii most blood feeding occurs indoors, when people are in the house and readily available as blood host, at certain times of the year blood feeding can occur outdoors, particularly when people spend the evening sitting outdoors [139]. Although An. arabiensis tends to feed outdoors, when hosts are scarce, they may also feed indoors [144,145,146]. On some occasions An. gambiae s.s. may feed on non-human hosts, including dogs and cattle [147], presumably as a fitness strategy. These events, however, are not common and in general humans are the preferred host for this species.
Indoor insecticide use
The use of insecticides for control of African malaria vectors through ITNs and indoor residual spraying (IRS) is directed at indoor-biting mosquitoes, mostly An. gambiae, An. coluzzii and An. funestus. It has been suggested that the extensive use of insecticides throughout sub-Saharan Africa may result in a behavioural change in the mosquitoes, leading to early-evening and outdoor biting when people are unprotected mostly outdoors where the mosquitoes are not exposed to the insecticides on interior walls or bed nets [20, 144, 148,149,150]. Whether such changes are due to behavioural traits selected for by the use of insecticides or simply normal variation in the behaviour of different populations needs further study. What is not debatable is that extensive use of ITNs has led to a collapse of An. gambiae s.s. populations in East Africa [151,152,153].
Indoor resting
After taking a blood meal, most female mosquitoes will rest indoors for two to three days while the blood meal is digested and eggs are developed. With most females, egg laying occurs after one meal, but with young mosquitoes may require two meals [44] (Fig. 3). Anopheles gambiae’s habit of resting indoors is a behaviour that is likely to increase the survival of a mosquito since the traditional thatched-roofed house protects mosquitoes against the extremely high temperatures experienced outdoors in the late afternoon and is an environment with relatively few predators. Metal-roofed houses, which are becoming increasingly common, are considerably hotter than thatched-roofed houses during the day and this results in decreased survival among indoor-resting mosquitoes [154, 155]. At hot times of the year, mosquitoes will move to the cooler darker and moister spaces at the bottom of the wall. Reduced mosquito survival in metal-roof houses may contribute to a decline in malaria transmission in sub-Saharan Africa.
In traditional houses, mosquitoes typically rest on the roof or base of the wall, particularly where water is stored in clay pots providing a suitable micro-climate. Blood feeding depresses mosquito activity for two to three days, and these mosquitoes are far less active than other gonotrophic stages [31, 156] resulting in most blood-feds remaining inside the house in which they fed, and only occasionally moving into neighbouring houses [157].
Oviposition
Eggs mature in blood-fed females within two to three days after a blood meal, after which they are ready to be laid on suitable oviposition sites. Gravid mosquitoes leave the house after dusk to find an aquatic habitat in which to oviposit. Female anophelines oviposit on still water, or on aquatic vegetation on the water surface. A median of 52 eggs are laid by individual mosquitoes, in the laboratory, and many practise skip overposition, laying their eggs in several containers [158]. Anopheles gambiae are generalists when it comes to selection of a site in which to lay their eggs and they are found in a wide range of habitats not just the easy-to-find hoofprint, footprint and tyre puddles. Indeed, in many places these sites are large semi-permanent or permanent water bodies in the shade or open to the sun, with clean and dirty water—although not highly odiferous water [159]. Experiments have shown that an ovipositing mosquito is attracted by water vapour [160] and by specific odours, like cedrol found in a large number of plants [161, 162] and nonane, a product from soil bacteria [163]. There are likely to be other chemical attractants and visual cues that also play a role in this vitally important part of the insect’s lifecycle. Future studies may lead to the development of novel ‘attract and kill strategies for malaria control.
Malaria transmission
In order for malaria transmission to occur a female An. gambiae needs to feed on a human that carries ‘male’ and ‘female’ (micro and macro-) Plasmodium gametocytes. Once in the stomach of the mosquito, the development of a flagella by the male gamete is triggered by xanthurenic acid which is a by-product of tryptophan metabolism. Exflagellation, where the eight motile gametes rupture the erythrocyte which contains them happens as a result of the drop in temperature in the mosquito. The micro-gametes move through the blood meal until they encounter a macro-gamete and fuse with it to form a motile, amoeba-like, oocyte. At this point in the life cycle the parasite is a diploid organism. At all other times it is haploid. The oocyte migrates through the midgut wall and there turns into an oocyst on the outer midgut wall. The extrinsic period of development depends critically on the environmental temperature [164]. For Plasmodium falciparum development of sporozoites in mosquitoes takes 11–12 days and for Plasmodium vivax 8–9 days at 26–27.5 °C. Plasmodium falciparum fails to develop at temperatures below 19 °C, whilst the lower limit for P. vivax is 15 °C [165]. Temperatures above 32 °C are lethal [158]. When the sporozoites are mature, oocysts rupture and sporozoites migrate through the haemocoel to the salivary glands after which the mosquito becomes infectious can pass on the parasites [166]. The percentage of female mosquitoes with sporozoites (the sporozoite rate) varies greatly, from 0 to 10% or even higher. Perhaps the factor that most affects the ability of a mosquito to be vector of disease is its survival rate. In order to become a malaria vector the mosquito has to survive through the extrinsic cycle of the parasite. This means that the mosquito, after taking an infectious meal, has to survive through four, or more, gonotrophic cycles before it will transmit.
At each phase of the gonotrophic cycle the mosquito faces different risks, each of which incurs a chance of dying. Thus, a defensive host may kill a mosquito attempting to blood feed, whilst the search for a suitable oviposition site and oviposition itself have their own risks and will depend on local conditions. For example, the risks to a mosquito that has to fly considerable distance to locate a potential oviposition site are much greater than for one where sites are to be found in close proximity to the feeding site. The risk of dying following feeding may also depend on the nature of the resting site. Mosquitoes that complete gonotrophic development inside traditional thatched roofed houses are in a more equitable environment than those that rest outdoors and so may be at less risk of desiccation. Thus, survival rate per gonotrophic cycle is important and has been estimated on a number of occasions using a variety of techniques, including dissection of the females’ ovaries to determine parous rates, mark-release-recapture experiments and estimates based on the delayed infection rate of mosquitoes [8]. In order to know the epidemiologically important figure of survival rate per day an estimate of the duration of the cycle needs to be obtained. Variations in both survival rate per cycle and cycle duration can have profound effects on the proportion of the population that might potentially become vectors. Importantly, small changes in daily survival rate can have huge consequences for the vectorial capacity of a vector [167].
On the other hand, as pointed out by [168,169,170], daily survival rates, determined by dissection, are remarkably similar between malaria vectors from different continents, which suggests that survival may be independent of the duration of the gonotrophic cycle. Cycle duration may, however, vary considerably with environmental factors [171].
In their meta-analysis of survival rate estimates according to methods used, Matthews, Bethel and Osei [172] obtained daily survival rates from dissections (vertical) of 0.83 (95% CI [0.80–0.86]), which was similar to the results obtained during population declines in the absence of recruitment [173]. Mark Release Recapture studies gave a lower value of 0.73 (95% CI [0.66–079]) whilst delayed infection rates (parasitological) gave 0.92, (CI [0.86–095]). Such differences result in large differences in estimates of vectorial capacity. Which of these methods provides the most accurate estimate is, however, not known. In many studies the effect of an intervention on survival is required, or estimates between different areas are needed. In such cases, for as long as similar methods are used for the estimates, absolute estimates are not needed.
Exploiting mosquito behaviour for surveillance and control
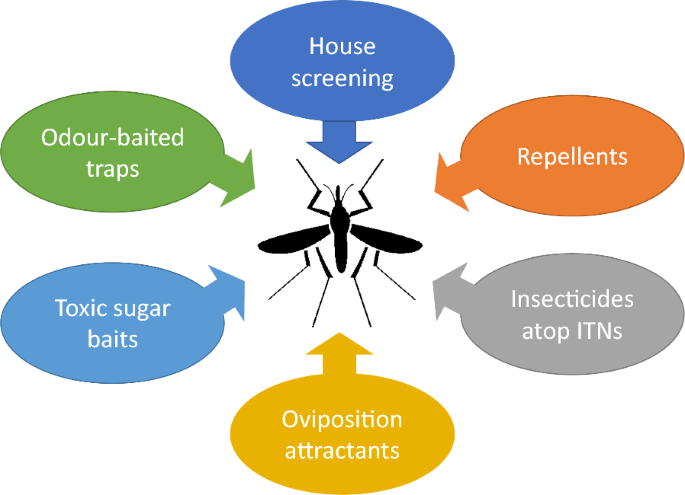
Over the past 20 years there has been an upsurge of interest in studying the behaviour and ecology of An. gambiae. How has this knowledge improved the control of this medically important insect? And can this be exploited to markedly enhance the toolbox of malaria control tools [174, 175]? Here are a number of potential new tools that exploit a knowledge of mosquito behaviour and may contribute to improved malaria control (Fig. 7).
-
1.
Screening houses
One of the most effective methods of malaria control is prevention of parasite transmission from mosquitoes to humans by preventing the entry of mosquitoes into houses [176]. When a mosquito cannot reach or find a human host, parasite transmission is effectively interrupted, and eventually malaria will die out while uninfected mosquitoes may continue to be present as they can feed elsewhere. Interruption of transmission can be achieved by making houses mosquito proof, by closing off all possible sites of entry such as eaves, windows, doors and even cracks in the wall. In such a mosquito proof house, mosquitoes cannot enter, and hence the occupants are protected from mosquito bites and their associated Plasmodium infections. There is a growing body of evidence suggesting that house modification (mainly screening) is protective against malaria [177].
Carbon dioxide is the principal cue attracting mosquitoes [76, 178], and in traditional houses carbon dioxide from human hosts is often leaking from a house through windows, doors and eaves. Understanding the movement of carbon dioxide from a house allows one to modify the design of a house to reduce carbon dixode leakage and produce a ‘stealth house’. For example, by increasing the ventilation in a house by installing three screened windows on opposite walls reduced indoor entry of An. gambiae by 95% [109]. Alternatively, when one makes all the walls air permeable, but not mosquito permeable, there was a 99% reduction in An. arabiensis compared with the comparator experimental hut with solid walls [90]. This finding is likely to results from a substantial drop in indoor carbon dioxide concentration, making it more difficult for mosquitoes to locate a host.
Limitations: the construction of mosquito-proof houses requires the householder to pay, unlike other interventions which are given freely. Behavioural changes are needed to keep doors closed at night.
-
2.
Repellents
Repellents have been used as a means to prevent mosquito bites for centuries. The sensory organs of mosquitoes are activated by the repellent compound(s) leading to aversion behaviour. Historically, botanical products were used for this purpose, and since the 1950s the chemical compound DEET has been used worldwide as a skin-treatment repelling mosquitoes [179, 180]. Novel repellents, to replace DEET, are in development at a large scale, with emphasis on human safety, residual activity and ease of application. Repellents can be used in different ways with promising results as tools for malaria prevention [181,182,183]. Long-lasting spatial repellents offer promise in the future as they can provide an area protection against mosquitoes. Recently, promising results were reported with transfluthrin-based passive emanator designed to release transfluthrin into the air. The emanators were placed indoors [184, 185].
Limitations: topical repellents are mostly effective as personal protection, and currently few products are available that provide community protection.
-
3.
Incorporating highly toxic insecticide on the rooftop of ITNs
This technology is based on the observation that, when bed nets are used, mosquitoes tend to first land on top of the net [186]. They are then exposed to an insecticide before flying off. As they spend longer on top of net than when hovering around the net, they are likely to pick up a larger dose of insecticide than when landing on the sides of the net. The discovery of increased mosquito activity at the top of the net could lead to bed nets produced with more human toxic insecticides applied to the top of the net.
Limitations: the availability of novel effective, safe, toxicants and the additional costs that require combinations of active ingredients and a net which is more complicated to construct.
-
4.
Oviposition attractants
Mosquitoes ready to oviposit, find oviposition sites based on selected olfactory cues. In Kenya, the compound cedrol was found to be highly attractive to gravid An. gambiae [187]. In Tanzania, the compound nonane in combination with soil microbials led to significantly more An. gambiae eggs laid in treated water bodies than in water bodies lacking this attractant [163]. These experiments demonstrate that baiting water bodies with selective attractive cues can be used as a strategy to reduce or even eliminate An. gambiae populations. This can be done when the oviposition attractants are combined with a pesticide such as temephos or Bacillus thuringiensis israeliensis which will kill all larvae when they emerge from the eggs [188]. Studies are now needed to examine the effect of these attractants on malaria prevalence and incidence over a large area.
Limitations: although oviposition attractants have the potential to reduce mosquito populations, studies are needed to demonstrate their feasibility. Competition with a plethora of natural sites may limit effectiveness.
-
5.
Toxic sugar baits
Sugar baits operate on the same principle as odour-baited traps: an attractive odour source is placed in the environment, attracting malaria vectors. By adding a toxic substance to the traps, usually a pyrethroid insecticide, mosquitoes making contact with the bait are killed. In this way, the adult mosquito population can be reduced. When critical density thresholds are reached, the malaria risk will have been significantly reduced as well [189]. A number of field trials measuring the efficacy of toxic sugar baits are currently in progress.
Potential limitations: competition with natural sugar sources may restrict the usefulness of TSBs and there is a need for long-lasting products.
-
6.
Odour-baited traps
Mosquito trapping is one of the main tools used for malaria surveillance. Routine trap catches can help design and target more effective interventions. From these collections, data on species composition, nutritional state, age distribution and associated Plasmodium infections are obtained and, are used for estimating malaria transmission risk [190]. Transmission risk captured as the number of infective bites likely to be received during a malaria season or year, often expressed as the entomological inoculation rate (EIR), is one of the main parameters on which decisions on malaria control are based: when transmission risk is high, interventions are needed to reduce new infections and malaria incidence. This is particularly important in urban areas where it is necessary to distinguish between local transmission and imported cases.
Odour-baited traps have been used to lower malaria transmission risk by removing the vector population with baited traps. By employing traps over a large area of several weeks, each day a fraction of the adult mosquitoes will be removed, thereby reducing the population of infectious mosquitoes continuously. Employing odour-baited traps in western Kenya, the percentage of people with malaria was reduced by more than 30% within 1 year [191]. In this study, carbon dioxide the most effective mosquito attractant, was not used. It is believed that addition of carbon dioxide or a carbon dioxide mimic may lead to strongly enhanced reduction of malaria than was achieved in this study. Odour-baited traps can also be used for mosquito surveillance, replacing the human-biting catch (HBC), which since surveillance began, has been the most-widely used trapping method [192].
Limitations: for this tool to be effective, strong attractants need to be identified, which preferably affect more than one Anopheles species. Also, durable and affordable traps should be readily available, that can be distributed across large areas. The generation of large quantities of carbon dioxide will contribute to global warming.
All these interventions have the potential to select for behavioural and, where there is an active ingredient involved, physiological resistance. Behavioural resistance may occur due to avoiding an active ingredient, or in the case of house screening, exophily.
Future behavioural research
Whilst there has been a considerable improvement in the understanding of the behaviour of An. gambiae, particularly over the past 20 years, there are a number of key areas of research where little, if anything, is known, some of which are highly relevant to malaria control. These include:
-
1.
Variability of behaviour
One of the major themes of this review is that there is considerable variation in behaviour between and within species of the An. gambiae complex. Yet, as far as the authors are aware, there have been no systematic reviews and meta-analyses of the behaviour of members An. gambiae in different parts of their range and over time. Such reviews are likely to provide important insights into the adaptability of this complex. A large number of publications on this behavioual variation have been written, and it will be interesting to explore if of concensus about a common trend can be reached. Climate change is an additional force that will affect mosquito behaviour, and development of parasites and their transmission. The huge variability in behaviour, as described in this paper, will no doubt be much affected by climate change and future studies will need to include this in understanding how this impacts Anopheles behaviour.
-
2.
Movement across the landscape
Long-distance movement of individual mosquitoes from their aquatic habitats to finding their host is poorly understood. The shape and texture of the landscape may provide natural barriers to dispersal or provide funnels to channel mosquitoes towards hosts. A deeper understanding may help us reduce malaria transmission in some areas.
-
3.
Light and vision
The role of visual attraction in host location is poorly understood in the field particularly for a nocturnal species like An. gambiae. Do mosquitoes navigate along intersections of light and dark, are they attracted to the outline of houses, and, if so does moonlight or electric lighting with tungsten or LED bulbs influence attraction or deterrence? With a rapid increase in electric lighting in parts of rural Africa it is important to understand how this may affect mosquito house entry and trapping with light traps.
-
4.
Behavioural barriers to gene flow
To help accelerate the deployment of gene drive mosquitoes in the field [193] it is important to understand the barriers to mating that occur in wild populations of mosquitoes and between laboratory-reared and wild mosquitoes. Whilst scientists may anticipate rapid spread of genes through a population of wild mosquitoes there are likely to be many natural behavioural barriers that restrict or prevent gene flow. For example, in a recent study in Burkina Faso, genetically-modified mosquitoes expressed reduced fitness and survival compared to wild mosquitoes [194].
-
5.
Male mosquito behaviour
Although understandably, most behavioural research has focused on adult females since only the female transmits malaria, there is little known bout the behaviour of adult males, apart from swarming and feeding. Although male mosquitoes use chemical cues for orientation, details about sensory behaviour of males are little known. Closer study of male behaviour may provide alternative targets for control.
-
6.
Evolutionary changes
Although it is well recognized that mosquitoes can adapt their behaviour in consequence of the large-scale use of insecticides [152], little consideration is given to how An. gambiae adapts to new environments. Africa has the fastest urban growth worldwide [195]. By 2050, the current population of 1.4 billion people is expected to increase by nearly one billion, most of these living in growing towns and cities. In some places, An. gambiae has already adapted to this new habitat. In the 1980s, a study in Accra, showed that An. gambiae had adapted to breeding in water-filled domestic containers and more polluted aquatic habitats [196]. In Dar es Salaam, a high proportion of transmission by An. gambiae sensu lato (s.l.) occurs outdoors [197]. Whether this represents a selected change in behaviour or one that results from houses with few entry points or both is uncertain. Nonetheless, it is important to detect changes in behaviour that may potentially favour malaria transmission. There is also the need to understand whether mosquitoes can adapt both behaviourally and physiological to extreme climate conditions [198].
-
7.
Alternatives to insecticide-based interventions
For the past 20 years, ITNs and IRS have been the main tools used for malaria control in sub-Saharan Africa. They have been remarkably successful at reducing the prevalence of malaria, but this control has stalled over the past 5–7 years and the number of malaria cases each year remains static. Increasingly, target mosquitoes develop resistance to the insecticides used, and massive research in the development of “novel” insecticides, and combination use of different insecticides, have been used to maintain the status quo of reduced Plasmodium parasite transmission. This race against the evolutionary strategy is likely to be lost, as it has done clearly in agriculture [199, 200]. Will the same strategy be used or the next 20 years or should one use alternative methods? With the growing urban populations, it is not feasible to deploy ITNs or IRS on a large scale due to poor compliance and some homes in informal settlements being too small to hang ITNs. Alternative forms of control need to be provided that are not reliant on synthetic insecticides, such as baited traps, push–pull systems.
Conclusion
This review illustrates the complex series of behaviours that female An. gambiae use on their journey from an aquatic habitat to a blood meal, resting and oviposition. It also underlines the huge variability in behaviours seen both between species and within species underscoring the adaptability of this mosquito to different environments. Adult mosquitoes navigate these behaviours by being exquisitely tuned to environmental cues, principally semiochemicals. A century of research has illuminated our understanding of the behaviour of this important insect and has allowed us to target vector control interventions where the mosquito is most vulnerable. Many of these interventions also apply to other mosquito vector species. Basic research on mosquito behaviour underpins applied studies on vector control and it is important that funding for this research continues to be supported in the future.
Data availability
Data availability All data supporting the findings of this study are available from corresponding author on reasonable request.
References
Boyd MF. Malariology: a comprehensive survey of all aspects of this group of diseases from a global standpoint, vol. 1. Saunders: University of Michigan; 1949. p. 1643.
Wernsdorfer WH, McGregor I. Malaria. Principles and Practice of Malariology. Vols 1 and 2. Churchill Livingstone, Edinburgh, 1988:20208 pp.
Gillies MT. Anopheline mosquitos: vector behaviour and bionomics. In: Wernsdorfer WH, McGregor I (eds.); Malaria - Principles and Practice of Malariology. Vol. 1. Churchill Livingstone, Edinburgh. 1988:453–85
White GB. Anopheles gambiae complex and disease transmission in Africa. Trans R Soc Trop Med Hyg. 1974;68:278–99.
Gillies MT, De Meillon B. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region). 2nd edn. S Afr Inst Med Res. 1968.
White BJ, Collins FH, Besansky NJ. Evolution of Anopheles gambiae in relation to humans and malaria. Annu Rev Ecol Evol Syst. 2011;42:111–32.
Godfray HCJ. Mosquito ecology and control of malaria. J Animal Ecol. 2013;82:15–25.
Charlwood JD. The Ecology of Malaria Vectors. Boca Raton: CRC Press, Taylor & Francis Group, LLC; 2020.
Coetzee M. Distribution of the African malaria vectors of the Anopheles gambiae complex. Am J Trop Med Hyg. 2004;70:103–4.
Barron M, Paupy C, Rahola N, Akone-Ella O, Ngangue MF, Wilson-Bahun T, et al. A new species in the major malaria vector complex sheds light on reticulated species evolution. Sci Rep. 2019;9:14753.
Niang EA, Konate L, Faye O, Diallo M, Dia I. Vector bionomics and malaria transmission in an area of sympatry of An. arabiensis, An. coluzzii and An. gambiae. Acta Trop. 2019;189:129–36.
Sinka ME, Golding N, Massey NC, Wiebe A, Huang Z, Hay SI, et al. Modelling the relative abundance of the primary African vectors of malaria before and after the implementation of indoor, insecticide-based vector control. Malar J. 2016;15:142.
Kamau L, Bennett KL, Ochomo E, Herren J, Agumba S, Otieno S, Omoke D, Matoke-Muhia D, Mburu D, Mwangangi J, Ramaita E, Juma EO, Mbobo C, Barasa S, Miles A. The Anopheles coluzzii range extends into Kenya: detection, insecticide resistance profiles and population genetic structure in relation to conspecific populations in West and Central Africa Malar J. 2024:122
Fornadel CM, Norris DE. Increased endophily by the malaria vector Anopheles arabiensis in southern Zambia and identification of digested blood meals. Am J Trop Med Hyg. 2010;83:838–42.
Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. S Afr Inst Med Res. 1987.
Sawadogo PS, Namountougou M, Toé KH, Rouamba J, Maïga H, Ouédraogo KR, et al. Swarming behaviour in natural populations of Anopheles gambiae and An. coluzzii: review of 4 years survey in rural areas of sympatry, Burkina Faso (West Africa). Acta Trop. 2014;132(Suppl):S42-52.
Charlwood JD, Kessy E, Yohannes K, Protopopoff N, Rowland M, LeClair C. Studies on the resting behaviour and host choice of Anopheles gambiae and An. arabiensis from Muleba, Tanzania. Med Vet Entomol. 2018;32:263–70.
Sinka M, Bangs M, Manguin S, Coetzee M, Mbogo C, Hemingway J, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2010;3:117.
Mwangangi JM, Mbogo CM, Orindi BO, Muturi EJ, Midega JT, Nzovu J, et al. Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malar J. 2013;12:13.
Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80.
Munga S, Minakawa N, Zhou G, Githeko AK, Yan G. Survivorship of immature stages of Anopheles gambiae s.l. (Diptera: culicidae) in natural habitats in western Kenya highlands. J Med Entomol. 2007;44:758–64.
Takken W, Smallegange R, Vigneau A, Johnston V, Brown M, Mordue-Luntz A, et al. Larval nutrition differentially affects adult fitness and Plasmodium development in the malaria vectors Anopheles gambiae and Anopheles stephensi. Parasit Vectors. 2013;6:345.
Takken W, Klowden MJ, Chambers GM. Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): the disadvantage of being small. J Med Entomol. 1998;35:639–45.
Bayoh MN, Lindsay SW. Temperature-related duration of aquatic stages of the Afrotropical malaria vector mosquito Anopheles gambiae in the laboratory. Med Vet Entomol. 2004;18:174–9.
Lyons CL, Coetzee M, Chown SL. Stable and fluctuating temperature effects on the development rate and survival of two malaria vectors, Anopheles arabiensis and Anopheles funestus. Parasit Vectors. 2013;6:104.
Reiter P, Jones DR. An eclosion timing mechanism in the mosquito Anopheles gambiae. Physiol Entomol. 2009;50:161–8.
Lindsay SW, Armstrong Schellenberg JR, Zeiler HA, Daly RJ, Salum FM, Wilkins HA. Exposure of Gambian children to Anopheles gambiae malaria vectors in an irrigated rice production area. Med Vet Entomol. 1995;9:50–8.
Gillies MT, Wilkes TJ. The effect of high fences on dispersal of some West-African mosquitoes (Diptera-Culicidae). Bull Entomol Res. 1978;68:401–8.
Snow WF. Studies of house-entering habits of mosquitoes in The Gambia, West Africa: experiments with prefabricated huts with various wall apertures. Med Vet Entomol. 1987;1:9–21.
Gillies MT, Wilkes TJ. Field experiments with a wind-tunnel on the flight speed of some West-African Mosquitoes (Diptera, Culicidae). Bull Entomol Res. 1981;71:65–70.
Jones MDR, Gubbins SJ. Changes in the circadian flight activity of the mosquito Anopheles gambiae in relation to insemination, feeding and oviposition. Physiol Entomol. 1978;3:213–20.
Guerra CA, Reiner RC, Perkins TA, Lindsay SW, Midega JT, Brady OJ, et al. A global assembly of adult female mosquito mark-release-recapture data to inform the control of mosquito-borne pathogens. Parasit Vectors. 2014;7:276.
Bogh C, Lindsay SW, Clarke SE, Dean A, Jawara M, Pinder M, et al. High spatial resolution mapping of malaria transmission risk in the Gambia, West Africa, using LANDSAT™ satellite imagery. Am J Trop Med Hyg. 2007;76:875–81.
Majambere S, Pinder M, Fillinger U, Ameh D, Conway DJ, Green C, et al. Is mosquito larval source management appropriate for reducing malaria in areas of extensive flooding in the Gambia? a cross-over intervention trial. Am J Trop Med Hyg. 2010;82:176–84.
Mutuku FM, Alaii JA, Bayoh MN, Gimnig JE, Vulule JM, Walker ED, et al. Distribution, description, and local knowledge of larval habitats of Anopheles gambiae s.l. in a village in western Kenya. Am J Trop Med Hyg. 2006;74:44–53.
Imbahale SS, Paaijmans KP, Mukabana WR, van Lammeren R, Githeko AK, Takken W. A longitudinal study on Anopheles mosquito larval abundance in distinct geographical and environmental settings in western Kenya. Malar J. 2011;10:81.
Lehmann T, Weetman D, Huestis DL, Yaro AS, Kassogue Y, Diallo M, et al. Tracing the origin of the early wet-season Anopheles coluzzii in the Sahel. Evol Appl. 2017;10:704–17.
Dao A, Yaro AS, Diallo M, Timbine S, Huestis DL, Kassogue Y, et al. Signatures of aestivation and migration in Sahelian malaria mosquito populations. Nature. 2014;516:387–90.
Atieli HE, Zhou G, Zhong D, Wang X, Lee MC, Yaro AS, et al. Wind-assisted high-altitude dispersal of mosquitoes and other insects in East Africa. J Med Entomol. 2023;60:698–707.
Parmakelis A, Russello MA, Caccone A, Marcondes CB, Costa J, Forattini OP, et al. Historical analysis of a near disaster: Anopheles gambiae in Brazil. Am J Trop Med Hyg. 2008;78:176–8.
Soper FL, Wilson DB. Anopheles gambiae in Brazil 1930 to 1940. Rockefeller Foundation, 1943.
Gillies MT. The recognition of age-groups within populations of Anopheles gambiae by the pre-gravid rate and the sporozoite rate. Ann Trop Med Parasitol. 1954;48:58–74.
Charlwood JD, Pinto J, Sousa CA, Ferreira C, Petrarca V, do E Rosario V. 'A mate for a meal'- Pre-gravid behaviour of female Anopheles gambiae from the islands of Sao Tome and Principe, West Africa. Malar J. 2003;2:9.
Scott TW, Takken W. Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol. 2012;28:114–21.
Manda H, Gouagna LC, Nyandat E, Kabiru EW, Jackson RR, Foster WA, et al. Discriminative feeding behaviour of Anopheles gambiae s.s. on endemic plants in western Kenya. Med Vet Entomol. 2007;21:103–11.
Nyasembe VO, Torto B. Volatile phytochemicals as mosquito semiochemicals. Phytochem Lett. 2014;8:196–201.
Nyasembe VO, Tchouassi DP, Pirk CWW, Sole CL, Torto B. Host plant forensics and olfactory-based detection in Afro-tropical mosquito disease vectors. PLoS Negl Trop Dis. 2018;12: e0006185.
Gary RE, Foster WA. Effects of available sugar on the reproductive fitness and vectorial capacity of the malaria vector Anopheles gambiae (Diptera: Culicidae). J Med Entomol. 2001;38:22–8.
Gouagna LC, Poueme RS, Dabire KR, Ouedraogo JB, Fontenille D, Simard F. Patterns of sugar feeding and host plant preferences in adult males of An. gambiae (Diptera: Culicidae). J Vector Ecol. 2010;35:267–76.
Foster WA, Takken W. Nectar-related vs. human-related volatiles: behavioural response and choice by female and male Anopheles gambiae (Diptera: Culicidae) between emergence and first feeding. Bull Entomol Res. 2004;94:145–57.
Klowden MJ. Endogenous factors regulating mosquito host-seeking behaviour. Ciba Foundation Symposium 200. Olfaction in mosquito-host interactions. Davis EE (ed.). 1996.
Omondi S, Kosgei J, Agumba S, Polo B, Yalla N, Moshi V, et al. Natural feeding rates of Anopheles mosquitoes collected by different methods in western Kenya. Sci Rep. 2022;12:20596.
Dahan YL, Koekemoer LL. Analysis of the genitalia rotation in the male Anopheles funestus (Diptera: Culicidae). Acta Trop. 2014;132:S20–5.
Gary RE, Cannon JW, Foster WA. Effect of sugar on male Anopheles gambiae mating performance, as modified by temperature, space, and body size. Parasit Vectors. 2009;2:19.
Nignan C, Niang A, Maiga H, Sawadogo SP, Poda BS, Gnankine O, et al. Comparison of swarming, mating performance and longevity of males Anopheles coluzzii between individuals fed with different natural fruit juices in laboratory and semi-field conditions. Malar J. 2020;19:173.
Charlwood JD, Jones MDR. Mating in the mosquito, Anopheles-gambiae s.l. II. Swarming behaviour. Physiol Entomol. 1980;5:315–20.
Butail S, Manoukis NC, Diallo M, Ribeiro JMC, Paley DA. The dance of male Anopheles gambiae in wild mating swarms. J Med Entomol. 2013;50:552–9.
Baeshen R. Swarming behavior in Anopheles gambiae (sensu lato): current knowledge and future outlook. J Med Entomol. 2022;59:56–66.
Charlwood JD, Pinto J, Sousa CA, Madsen H, Ferreira C. do Rosario VE: The swarming and mating behaviour of Anopheles gambiae s.s. (Diptera: Culicidae) from São Tomé Island. J Vector Ecol. 2002;27:178–83.
Poda SB, Nignan C, Gnankiné O, Dabiré RK, Diabaté A, Roux O. Sex aggregation and species segregation cues in swarming mosquitoes: role of ground visual markers. Parasit Vectors. 2019;12:589.
Downes JA. The swarming and mating flight of Diptera. Ann Rev Entomol. 1969;14:271–98.
Charlwood JD, Thompson R, Madsen H. Observations on the swarming and mating behaviour of Anopheles funestus from southern Mozambique. Malar J. 2003;2:2.
Cator LJ, Ng’Habi KR, Hoy RR, Harrington LC. Sizing up a mate: variation in production and response to acoustic signals in Anopheles gambiae. Behavior Ecol. 2010;21:1033–9.
Charlwood JD, Jones MDR. Mating behaviour in the mosquito, Anopheles gambiae s.l. I. Close range and contact behaviour. Physiol Entomol. 1979;4:111–20.
Somers J, Georgiades M, Su MP, Bagi J, Andres M, Alampounti A, et al. Hitting the right note at the right time: circadian control of audibility in Anopheles mosquito mating swarms is mediated by flight tones. Sci Adv. 2022;8:eabl4844.
Sterelny K, Griffiths P (1999) Sex and death. An introduction to philosophy of biology. Chicago: University of Chicago Press.
Cator L, Hoy RR, Harrington LC. Harmonic convergence and the sexy sons hypothesis in Aedes aegypti. Am J Trop Med Hyg. 2010;83:181.
Gibson G, Warren B, Russell IJ. Humming in tune: sex and species recognition by mosquitoes on the wing. J Assoc Res Otolaryngol. 2010;11:527–40.
Diabaté A, Yaro AS, Dao A, Diallo M, Huestis DL, Lehmann T. Spatial distribution and male mating success of Anopheles gambiae swarms. BMC Evol Biol. 2011;11:184.
Charlwood JD. Swarming and mate selection in Anopheles gambiae mosquitoes (Diptera: Culicidae). J Med Entomol. 2023;60:857–64.
Dao A, Adamou A, Yaro AS, Maïga HM, Kassogue Y, Traoré SF, et al. Assessment of alternative mating strategies in Anopheles gambiae: does mating occur indoors? J Med Entomol. 2008;45:643–52.
Nambunga IH, Msugupakulya BJ, Hape EE, Mshani IH, Kahamba NF, Mkandawile G, et al. Wild populations of malaria vectors can mate both inside and outside human dwellings. Parasit Vectors. 2021;14:514.
Gillies MT. A new character for the recognition of nulliparous females of Anopheles gambiae. Bull World Health Organ. 1956;15:451–9.
Klowden MJ. Sexual receptivity in Anopheles gambiae mosquitoes: absence of control by male accessory gland substances. J Insect Physiol. 2001;47:661–6.
Gabrieli P, Smidler A, Catteruccia F. Engineering the control of mosquito-borne infectious diseases. Genome Biol. 2014;15:535.
Gillies MT. The role of carbon dioxide in host-finding by mosquitoes (Diptera:Culicidae): a review. Bull Entomol Res. 1980;70:525–32.
Lucas-Barbosa D, DeGennaro M, Mathis A, Verhulst NO. Skin bacterial volatiles: propelling the future of vector control. Trends Parasitol. 2022;38:15–22.
Showering A, Martinez J, Benavente ED, Gezan SA, Jones RT, Oke C, et al. Skin microbiome alters attractiveness to Anopheles mosquitoes. BMC Microbiol. 2022;22:98.
van Loon JJA, Smallegange RC, Bukovinszkiné-Kiss G, Jacobs F, De Rijk M, Mukabana WR, et al. Mosquito attraction: crucial role of carbon dioxide in formulation of a five-component blend of human-derived volatiles. J Chem Ecol. 2015;41:567–73.
Verhulst NO, Umanets A, Weldegergis BT, Maas JPA, Visser TM, Dicke M, et al. Do apes smell like humans? The role of skin bacteria and volatiles of primates in mosquito host selection. J Exp Biol. 2018;221:jeb185959.
Montell C, Zwiebel LJ. Mosquito sensory systems. In: Raikhel AS (eds) Progress in mosquito research. Adv Insect Physiol. 2016;51:293–328.
Konopka JK, Task D, Afify A, Raji J, Deibel K, Maguire S, et al. Olfaction in Anopheles mosquitoes. Chem Senses. 2021;46:bjab021.
Suh E, Bohbot JD, Zwiebel LJ. Peripheral olfactory signaling in insects. Curr Opin Insect Sci. 2014;6:86–92.
Bidlingmayer WL. The influence of environmetal factors and physiological stage on flight patterns of mosquitoes taken in the vehicle aspirator and truck, suction, bait and New Jersey light traps. J Med Entomol. 1974;2:119–46.
Bidlingmayer WL, Hem DG. The range of visual attraction and the effect of competitive visual attractants upon mosquito (Diptera: Culicidae) flight. Bull Entomol Res. 1980;70:321–42.
Bidlingmayer WL. Mosquito flight paths in relation to environment. 1. Illumination levels, orientation, and resting areas. Ann Entomol Soc Am. 1971;64:1121–31.
Bidlingmayer WL. Mosquito flight paths in relation to environment—effect of vertical and horizontal visual barriers. Ann Entomol Soc Am. 1975;68:51–7.
Bidlingmayer WL, Hem DG. Mosquito (Diptera, Culicidae) flight behavior near conspicuous objects. Bull Entomol Res. 1979;69:691–5.
Gillies MT, Wilkes TJ. Responses of host-seeking Mansonia and Anopheles mosquitoes (Diptera: culicidae) in West Africa to visual features of a target. J Med Entomol. 1982;19:68–71.
Mmbando AS, Bradley J, Kazimbaya D, Kasubiri R, Knudsen J, Siria D, et al. The effect of light and ventilation on house entry by Anopheles arabiensis sampled using light traps in Tanzania: an experimental hut study. Malar J. 2022;21:36.
Odetoyinbo JA. Preliminary investigation on the use of a light-trap for sampling malaria vectors in the Gambia. Bull World Health Organ. 1969;40:547–60.
Charlwood JD, Rowland M, Protopopoff N, Le Clair C. The Furvela tent-trap Mk 1.1 for the collection of outdoor biting mosquitoes. PeerJ. 2017;5:e3848.
Fornadel CM, Norris LC, Norris DE. Centers for Disease Control light traps for monitoring Anopheles arabiensis human biting rates in an area with low vector density and high insecticide-treated bed net use. Am J Trop Med Hyg. 2010;83:838–42.
Olanga EA, Okal MN, Mbadi PA, Kokwaro ED, Mukabana WR. Attraction of Anopheles gambiae to odour baits augmented with heat and moisture. Malar J. 2010;9:6.
Hawkes FM, Dabire RK, Sawadogo SP, Torr SJ, Gibson G. Exploiting Anopheles responses to thermal, odour and visual stimuli to improve surveillance and control of malaria. Sci Rep. 2017;7:17283.
De Jong R, Knols BGJ. Selection of biting sites by mosquitoes. In: Olfaction in mosquito host interactions. Bock GR, Cardew G (eds). CIBA Found Symp. 1996;200:89–103.
Braack L, Hunt R, Koekemoer LL, Gericke A, Munhenga G, Haddow AD, et al. Biting behaviour of African malaria vectors: 1. Where do the main vector species bite on the human body? Parasit Vectors. 2015;8:76.
Lindsay SW, Snow RW. The trouble with eaves; house entry by vectors of malaria. Trans R Soc Trop Med Hyg. 1988;82:645–6.
Njie M, Dilger E, Lindsay SW, Kirby MJ. Importance of eaves to house entry by Anopheline, but not Culicine, mosquitoes. J Med Entomol. 2009;46:505–10.
Mshamu S, Mmbando A, Meta J, Bradley J, Bojstrup TC, Day NPJ, et al. Assessing the impact of a novel house design on the incidence of malaria in children in rural Africa: study protocol for a household-cluster randomized controlled superiority trial. Trials. 2022;23:519.
Jatta E, Jawara M, Bradley J, Jeffries D, Kandeh B, Knudsen JB, et al. How house design affects malaria mosquito density, temperature, and relative humidity: an experimental study in rural Gambia. Lancet Planet Health. 2018;2:e498–508.
Haddow AJ. The mosquito fauna and climate of native huts at Kisumu, Kenya. Bull Entomol Res. 1942;33:91–142.
Kaindoa EW, Mkandawile G, Ligamba G, Kelly-Hope LA, Okumu FO. Correlations between household occupancy and malaria vector biting risk in rural Tanzanian villages: implications for high-resolution spatial targeting of control interventions. Malar J. 2016;15:199.
Spitzen J, Koelewijn T, Mukabana WR, Takken W. Visualization of house-entry behaviour of malaria mosquitoes. Malar J. 2016;15:233.
Carrasco-Tenezaca M, Jawara M, Abdi MY, Bradley J, Brittain OS, Ceesay S, et al. The relationship between house height and mosquito house entry: an experimental study in rural Gambia. J R Soc Interface. 2021;18:20210256.
Carrasco-Tenezaca M, Jawara M, Lee DSH, Holmes MS, Ceesay S, McCall P, et al. Effect of passive and active ventilation on malaria mosquito house entry and human comfort: an experimental study in rural Gambia. J R Soc Interface. 2023;20:20220794.
Charlwood JD, Pinto J, Ferrara PR, Sousa CA, Ferreira C, Gil V, et al. Raised houses reduce mosquito bites. Malar J. 2003;2:45.
Parker JEA, Angarita-Jaimes N, Abe M, Towers CE, Towers D, McCall PJ. Infrared video tracking of Anopheles gambiae at insecticide-treated bed nets reveals rapid decisive impact after brief localised net contact. Sci Rep. 2015;5:13392.
Jatta E, Carrasco-Tenezaca M, Jawara M, Bradley J, Ceesay S, D’Alessandro U, et al. Impact of increased ventilation on indoor temperature and malaria mosquito density: an experimental study in The Gambia. J R Soc Interface. 2021;18:178.
Carnevale P, Frezil JL, Bosseno MF, Le Pont F, Lancien J. Etude de l’agressivité d’Anopheles gambiae A en fonction de l’age et du sexe des sujets humains. Bull World Health Organ. 1978;56:147–54.
Lindsay SW, Adiamah JH, Miller JE, Pleass RJ, Armstrong JR. Variation in attractiveness of human subjects to malaria mosquitoes (Diptera: Culicidae) in The Gambia. J Med Entomol. 1993;30:368–73.
Knols BGJ, De Jong R, Takken W. Differential attractiveness of isolated humans to mosquitoes in Tanzania. Trans R Soc Trop Med Hyg. 1995;89:604–6.
Fernandez-Grandon GM, Gezan SA, Armour JAL, Pickett JA, Logan JG. Heritability of attractiveness to mosquitoes. PLoS ONE 215;10(4): e0122716.
Port GR, Boreham PFL, Bryan JH. The relationship of host size to feeding by mosquitoes of the Anopheles gambiae Giles complex (Diptera: Culicidae). Bull Entomol Res. 1980;70:133–44.
Lindsay S, Ansell J, Selman C, Cox V, Hamilton K, Walraven G. Effect of pregnancy on exposure to malaria mosquitoes. Lancet. 2000;355:1972.
Ansell J, Hamilton KA, Pinder M, Walraven GE, Lindsay SW. Short-range attractiveness of pregnant women to Anopheles gambiae mosquitoes. Trans R Soc Trop Med Hyg. 2002;96:113–6.
Verhulst NO, Andriessen R, Groenhagen U, Kiss GB, Schulz S, Takken W, et al. Differential attraction of malaria mosquitoes to volatile blends produced by human skin bacteria. PLoS ONE. 2010;5: e15829.
Busula AO, Takken W, De Boer JG, Mukabana WR, Verhulst NO. Variation in host preferences of malaria mosquitoes is mediated by skin bacterial volatiles. Med Vet Entomol. 2017;31:320–6.
Lacroix R, Mukabana WR, Gouagna LC, Koella JC. Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol. 2005;3: e298.
Busula AO, Bousema T, Mweresa CK, Masiga D, Logan JG, Sauerwein RW, et al. Gametocytemia and attractiveness of Plasmodium falciparum-infected Kenyan children to Anopheles gambiae mosquitoes. J Infect Dis. 2017;216:291–5.
Robinson A, Busula AO, Voets MA, Beshir KB, Caulfield JC, Powers SJ, et al. Plasmodium-associated changes in human odor attract mosquitoes. Proc Natl Acad Sci USA. 2018;115:E4209–18.
Carnaghi M, Belmain SR, Hopkins RJ, Hawkes FM. Multimodal synergisms in host stimuli drive landing response in malaria mosquitoes. Sci Rep. 2021;11:7379.
Zembere K, Chirombo J, Nasoni P, McDermott DP, Tchongwe-Divala L, Hawkes FM, et al. The human-baited host decoy trap (HDT) is an efficient sampling device for exophagic Anopheles arabiensis within irrigated lands in southern Malawi. Sci Rep. 2022;12:3428.
Gordon RM, Lumsden WHR. A study of the behaviour of the mouth-parts of mosquitoes when taking up blood from living tissue; together with some observations on the ingestion of microfilariae. Ann Trop Med Parasitol. 1939;33:259–78.
Robinson GG. The mouthparts and their function in the female mosquito, Anopheles maculipennis. Parasitology. 1939;31:212–42.
Griffiths RB, Gordon RM. An apparatus which enables the process of feeding by mosquitoes to be observed in the tissues of a live rodent; together with an account of the ejection of saliva and its significance in malaria. Ann Trop Med Parasitol. 1952;46:311–9.
Christophers SR. The yellow fever mosquito. Cambridge: Cambridge University Press; 1960.
Ribeiro JMC. Blood-feeding arthropods - live syringes or invertebrate pharmacologists. Infect Agents Dis. 1995;4:143–52.
Clements AN. The biology of mosquitoes. London: Chapman & Hall; 1992.
Choo YM, Buss GK, Tan KM, Leal WS. Multitasking roles of mosquito labrum in oviposition and blood feeding. Front Physiol. 2015;6:306.
Lahondère C, Lazzari CR. Mosquitoes cool down during blood feeding to avoid overheating. Curr Biol. 2012;22:40–5.
Ribeiro JMC, Rossignol PA, Spielman A. Role of mosquito saliva in blood vessel location. J Exp Biol. 1984;108:1–7.
Rossignol PA, Ribeiro JM, Spielman A. Increased biting rate and reduced fertility in sporozoite-infected mosquitoes. Am J Trop Med Hyg. 1986;35:277–9.
Koella JC, Sorensen FL, Anderson RA. The malaria parasite, Plasmodium falciparum, increases the frequency of multiple feeding of its mosquito vector, Anopheles gambiae. Proc Biol Sci. 1998;265:763–8.
Lindsay SW, Campbell H, Adiamah JH, Greenwood AM, Bangali JE, Greenwood BM. Malaria in a peri-urban area of The Gambia. Ann Trop Med Parasitol. 1990;84:553–62.
Kabbale FG, Akol AM, Kaddu JB, Onapa AW. Biting patterns and seasonality of Anopheles gambiae sensu lato and Anopheles funestus mosquitoes in Kamuli District, Uganda. Parasit Vectors. 2013;6:340.
Sherrard-Smith E, Hogan AB, Hamlet A, Watson OJ, Whittaker C, Winskill P, et al. The potential public health consequences of COVID-19 on malaria in Africa. Nat Med. 2020;26:1411–6.
Yohannes M, Haile M, Ghebreyesus TA, Witten KH, Getachew A, Byass P, et al. Can source reduction of mosquito larval habitat reduce malaria transmission in Tigray, Ethiopia? Trop Med Int Health. 2005;10:1274–85.
Mburu MM, Mzilahowa T, Amoah B, Chifundo D, Phiri KS, van den Berg H, et al. Biting patterns of malaria vectors of the lower Shire valley, southern Malawi. Acta Trop. 2019;197: 105059.
Bockarie MJ, Alexander N, Bockarie F, Ibam E, Barnish G, Alpers M. The late biting habit of parous Anopheles mosquitoes and pre-bedtime exposure of humans to infective female mosquitoes. Trans R Soc Trop Med Hyg. 1996;90:23–5.
Charlwood JD, Pinto J, Sousa CA, Ferreira C, Gil V, Do Rosario VE. Mating does not affect the biting behaviour of Anopheles gambiae from the islands of Sao Tome and Principe, West Africa. Ann Trop Med Parasitol. 2003;97:751–6.
Sangbakembi-Ngounou C, Costantini C, Longo-Pendy NM, Goagouni C, Akoni-Ella O, Rahola N, et al. Diurnal biting of malaria mosquitoes in the Central African Republic indicates residual transmission may be “out of control.” Proc Natl Acad Sci USA. 2022;119: e2104282119.
Lefevre T, Gouagna LC, Dabire KR, Elguero E, Fontenille D, Costantini C, et al. Evolutionary lability of odour-mediated host preference by the malaria vector Anopheles gambiae. Trop Med Int Health. 2009;14:228–36.
Killeen GF, Govella NJ, Lwetoijera DW, Okumu FO. Most outdoor malaria transmission by behaviourally-resistant Anopheles arabiensis is mediated by mosquitoes that have previously been inside houses. Malar J. 2016;15:225.
Tirados I, Costantini C, Gibson G, Torr SJ. Blood-feeding behaviour of the malarial mosquito Anopheles arabiensis: implications for vector control. Med Vet Entomol. 2006;20:425–37.
Mburu MM, Zembere K, Mzilahowa T, Terlouw AD, Malenga T, van den Berg H, et al. Impact of cattle on the abundance of indoor and outdoor resting malaria vectors in southern Malawi. Malar J. 2021;20:353.
Sousa CA, Pinto J, Almeida PG, Ferreira C, Do Rosário VEJ, Charlwood JD. J Med Entomol. 2001;38:122–5.
Gatton ML, Chitnis N, Churcher T, Donnelly MJ, Ghani AC, Godfray HCJ, et al. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution. 2013;67:1218–30.
Kreppel KS, Viana M, Main BJ, Johnson PCD, Govella NJ, Lee Y, et al. Emergence of behavioural avoidance strategies of malaria vectors in areas of high LLIN coverage in Tanzania. Sci Rep. 2020;10:14527.
Perugini E, Guelbeogo WM, Calzetta M, Manzi S, Virgillito C, Caputo B, et al. Behavioural plasticity of Anopheles coluzzii and Anopheles arabiensis undermines LLIN community protective effect in a Sudanese-savannah village in Burkina Faso. Parasit Vectors. 2020;13:277.
Killeen GF, Seyoum A, Sikaala C, Zomboko AS, Gimnig JE, Govella NJ, et al. Eliminating malaria vectors. Parasit Vectors. 2013;6:172.
Bayoh MN, Mathias D, Odiere M, Mutuku F, Kamau L, Gimnig J, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J. 2010;9:62.
Finda MF, Limwagu AJ, Ngowo HS, Matowo NS, Swai JK, Kaindoa E, et al. Dramatic decreases of malaria transmission intensities in Ifakara, south-eastern Tanzania since early 2000s. Malar J. 2018;17:362.
Lindsay SW, Jawara M, Mwesigwa J, Achan J, Bayoh N, Bradley J, et al. Reduced mosquito survival in metal-roof houses may contribute to a decline in malaria transmission in sub-Saharan Africa. Sci Rep. 2019;9:7770.
Lindsay SW, Davies M, Alabaster G, Altamirano H, Jatta E, Jawara M, et al. Recommendations for building out mosquito-transmitted diseases in sub-Saharan Africa: the DELIVER mnemonic. Philos Trans R Soc Lond B Biol Sci. 2021;376:20190814.
Takken W, Van Loon JJA, Adam W. Inhibition of host-seeking response and olfactory responsiveness in Anopheles gambiae following blood feeding. J Insect Physiol. 2001;47:303–10.
Boreham PFL, Port GR. The distribution and movement of engorged females of Anopheles gambiae Giles (Diptera: Culicidae) in a Gambian village. Bull Ent Res. 1982;72:489–95.
Okal MN, Lindh JM, Torr SJ, Masinde E, Orindi B, Lindsay SW, et al. Analysing the oviposition behaviour of malaria mosquitoes: design considerations for improving two-choice egg count experiments. Malar J. 2015;14:250.
Fillinger U, Lindsay SW. Larval source management for malaria control in Africa: myths and reality. Malar J. 2011;10:353.
Okal MN, Francis B, Herrera-Varela M, Fillinger U, Lindsay SW. Water vapour is a pre-oviposition attractant for the malaria vector Anopheles gambiae sensu stricto. Malar J. 2013;12:365.
Lindh JM, Okal MN, Herrera-Varela M, Borg-Karlson A-K, Torto B, Lindsay SW, et al. Discovery of an oviposition attractant for gravid malaria vectors of the Anopheles gambiae species complex. Malar J. 2015;14:119.
Eneh LK, Fillinger U, Karlson AKB, Rajarao GK, Lindh J. Anopheles arabiensis oviposition site selection in response to habitat persistence and associated physicochemical parameters, bacteria and volatile profiles. Med Vet Entomol. 2019;33:56–67.
Schoelitsz B, Mwingira V, Mboera LEG, Beijleveld H, Koenraadt CJM, Spitzen J, et al. Chemical mediation of oviposition by Anopheles mosquitoes: a push-pull system driven by volatiles associated with larval stages. J Chem Ecol. 2020;46:397–409.
MacDonald G. The epidemiology and control of malaria. London: Oxford University Press; 1957.
Warrell DA, Gilles HM. Bruce-Chwatt’s Essential Malariology. 4th ed. Boca Raton, Florida, USA: CRC Press; 2002.
Josling GA, Llinas M. Sexual development in Plasmodium parasites: knowing when it’s time to commit. Nat Rev Microbiol. 2015;13:573–87.
Garrett-Jones C, Shidrawi GR. Malaria vectorial capacity of a population of Anopheles gambiae. Bull World Health Organ. 1969;40:531–45.
Hii JLK, Birley MH, Sang VY. Estimation of survival rate and oviposition interval of Anopheles balabacensis mosquitos from mark recapture experiments in Sabah, Malaysia. Med Vet Entomol. 1990;4:135–40.
Hii JL. Evidence for the existence of genetic variability in the tendency of Anopheles balabacensis to rest in houses and to bite man, Southeast Asian. J Trop Med Public Health. 1985;16:173–82.
Hii JL, Kan S, Vun YS, Chin KF, Lye MS, Mak JW, et al. Anopheles flavirostris incriminated as a vector of malaria and Bancroftian filariasis in Banggi Island, Sabah, Malaysia. Trans R Soc Trop Med Hyg. 1985;79:677–80.
Beck-Johnson LM, Nelson WA, Paaijmans KP, Read AF, Thomas MB, Bjornstad ON. The effect of temperature on Anopheles mosquito population dynamics and the potential for malaria transmission. PLoS ONE. 2013;8: e79276.
Matthews J, Bethel A, Osei G. An overview of malarial Anopheles mosquito survival estimates in relation to methodology. Parasit Vectors. 2020;13:233.
Charlwood JD, Birley MH, Dagaro H, Paru R, Holmes PR. Assessing survival rate of Anopheles farauti (Diptera: Culicidae) from New Guinea. J Anim Ecol. 1985;54:1003–16.
Killeen GF, Masalu JP, Chinula D, Fotakis EA, Kavishe DR, Malone D, et al. Control of malaria vector mosquitoes by insecticide-treated combinations of window screens and eave baffles. Emerg Infect Dis. 2017;23:782–9.
Williams YA, Tusting LS, Hocini S, Graves PM, Killeen GF, Kleinschmidt I, et al. Expanding the vector control toolbox for malaria elimination: a systematic review of the evidence. Adv Parasitol. 2018;99:345–79.
WHO. Guidelines for malaria. Geneva: World Health Organization; 2023.
Fox T, Furnival-Adams J, Chaplin M, Napier M, Olanga EA. House modifications for preventing malaria. Cochrane Database Syst Rev. 2022;2022: CD013398.
Mboera LEG, Takken W. Carbon dioxide chemotropism in mosquitoes (Diptera: Culicidae) and its potential in vector surveillance and management programmes. Med Vet Entomol. 1997;85:355–68.
Bohbot JD, Dickens JC. Insect repellents: modulators of mosquito odorant receptor activity. PLoS ONE. 2010;5: e12138.
Afify A, Potter CJ. Insect repellents mediate species-specific olfactory behaviours in mosquitoes. Malar J. 2020;19:127.
Mmbando AS, Batista EPA, Kilalangongono M, Finda MF, Mwanga EP, Kaindoa EW, et al. Evaluation of a push-pull system consisting of transfluthrin-treated eave ribbons and odour-baited traps for control of indoor- and outdoor-biting malaria vectors. Malar J. 2019;18:87.
Masalu JP, Finda M, Killeen GF, Ngowo HS, Pinda PG, Okumu FO. Creating mosquito-free outdoor spaces using transfluthrin-treated chairs and ribbons. Malar J. 2020;19:109.
Njoroge MM, Hiscox A, Saddler A, Takken W, van Loon JJA, Fillinger U. Less is more: repellent-treated fabric strips as a substitute for full screening of open eave gaps for indoor and outdoor protection from malaria mosquito bites. Parasit Vectors. 2022;15:259.
Syafruddin D, Asih PBS, Rozi IE, Permana DH, Nur Hidayati AP, Syahrani L, et al. Efficacy of a spatial repellent for control of malaria in indonesia: a cluster-randomized controlled trial. Am J Trop Med Hyg. 2020;103:344–58.
Achee NL, Perkins TA, Moore SM, Liu F, Sagara I, Van Hulle S, et al Spatial repellents: the current roadmap to global recommendation of spatial repellents for public health use. Curr Res Parasitol Vector Borne Dis. 2022;9:100107.
Jones J, Murray GPD, McCall PJ. A minimal 3D model of mosquito flight behaviour around the human baited bed net. Malar J. 2021;20:24.
Eneh LK, Saijo H, Borg-Karlson AK, Lindh JM, Rajarao GK. Cedrol, a malaria mosquito oviposition attractant is produced by fungi isolated from rhizomes of the grass Cyperus rotundus. Malar J. 2016;15:478.
Mwingira V, Dicke M, Takken W. Exploiting the chemical ecology of mosquito-oviposition surveillance and control: a review. J Vect Ecol. 2020;12:155–79.
Traore MM, Junnila A, Traore SF, Doumbia S, Revay EE, Kravchenko VD, et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malar J. 2020;19:72.
Amoah B, McCann RS, Kabaghe AN, Mburu M, Chipeta MG, Moraga P, et al. Identifying Plasmodium falciparum transmission patterns through parasite prevalence and entomological inoculation rate. Elife. 2021;10: e65682.
Homan T, Hiscox A, Mweresa CK, Masiga D, Mukabana WR, Oria P, et al. The effect of mass mosquito trapping on malaria transmission and disease burden (SolarMal): a stepped-wedge cluster-randomised trial. Lancet. 2016;388:1193–201.
Hawkes F, Manin BO, Ng SH, Torr SJ, Drakeley C, Chua TH, Ferguson HM. Evaluation of electric nets as means to sample mosquito vectors host-seeking on humans and primates. Parasit Vectors. 2017;10:338.
James SL, Dass B, Quemada H. Regulatory and policy consideration for the implementation of gene drive-modified mosquitos to prevent malaria transmission. Transgenic Res. 2023;32:17–32.
Yao FA, Millogo AA, Epopa PS, North A, Noulin F, Dao K, et al. Mark-release-recapture experiment in Burkina Faso demonstrates reduced fitness and dispersal of genetically-modified sterile malaria mosquitoes. Nat Commun. 2022;13:796.
United Nations. World Urbanization Prospects. The 2018 Revision. 2019:103pp.
Chinery WA. Effects of ecological changes on the malaria vector Anopheles funestus and the Anopheles gambiae complex of mosquitoes in Accra, Ghana. J Trop Med Hyg. 1984;87:75–81.
Geissbühler Y, Chaki P, Emidi B, Govella NJ, Shirima R, Mayagaya V, et al. Interdependence of domestic malaria prevention measures and mosquito-human interactions in urban Dar es Salaam, Tanzania. Malar J. 2007;6:126.
Sternberg ED, Thomas MB. Local adaptation to temperature and the implications for vector-borne diseases. Trends Parasitol. 2014;30:115–22.
Thomas MB, Godfray HCJ, Read AF, van den Berg H, Tabashnik BE, van Lenteren JC, et al. Lessons from agriculture for the sustainable management of malaria vectors. PLoS Med. 2012;9: e1001262.
Sternberg ED, Thomas MB. Insights from agriculture for the management of insecticide resistance in disease vectors. Evol Appl. 2018;11:404–14.
Patin E, Qiuntana-Murci L. The demographic and adaptive history of central African hunter-gatherers and farmers. Curr Opin Genet Develop. 2018;53:90–7.
Patin E, Siddle KJ, Laval G, Quach H, Harmant C, Becker N, Froment A, et al. The impact of agricultural emergence on the genetic history of African rainforest hunter-gatherers and agriculturalists. Nat Commun. 2014;5:3163.
Aimé C, Laval G, Patin E, Verdu P, Ségurel L, Chaix R, et al. Human genetic data reveal contrasting demographic patterns between sedentary and nomadic populations that predate the emergence of farming. Mol Biol Evol. 2013;30:2629–44.
Snow RW, Trape JF, Marsh K. The past, present and future of childhood malaria mortality in Africa. Trends Parasitol. 2001;17:593–7.
Livi-Bacci M. A Concise History of World Population, 6th Edn. Wiley-Blackwell; 2017.
Manning P. The impact of agricultural emergence on the genetic history of African rainforest hunter-gatherers and agriculturalists. In: African Economic History Conference. Vancouver, 2013:13.
Tabutin D, Schoumaker B. The demography of sub-saharan Africa from the 1950s to the 2000s: a survey of changes and a statistical assessment. Population. 2004;59:97.
Thornton JK. Demography and history in the kingdom of Kongo, 1550–1750. J Afr Hist. 1977;18:507–30.
WHO. World malaria report 2022. Geneva: World Health Organization; 2022.
Bolt J, van Zanden JL. Maddison Project Database 2020. Groningen Growth and Development Centre, Groningen, 2021.
Acknowledgements
We thank Maureen Coetzee for advice on the Anopheles gambiae species complex. Jeroen Spitzen is thanked for his comments on the house-entry section.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Estimating the number of malaria deaths attributed to Anopheles gambiae s.l.
Whilst it is impossible to make precise estimates of the number of deaths from malaria attributed to An. gambiae, it is likely to be considerable. It is estimated that about 241 million children under five years old have died from this disease in sub-Saharan Africa between 3000 BC and 2000 AD, making it the world’s greatest assassin. Part of the reason for this enormous attrition is because, unusually for a mosquito, An. gambiae evolved a propensity for feeding on people, presumably when humankind began cultivating crops, becoming relatively sedentary, with an increase in group size and sleeping in the same place for several years, making it more likely that vector populations increased in abundance increasing malaria. Shifting agriculture was the earliest form of agriculture practised in the forests of Gabon around 5000 years ago [201,202,203]. In this system the forest was cleared to grow crops for two to three years before moving on when soil nutrients were depleted. When the people moved to neighbouring forests, mosquitoes followed with them as humans formed a reliable food source, unlike wildlife which were relatively scarce in the rainforest with larger home ranges [203]. It is likely this would have led to an increase in malaria in early ancestral populations.
The accumulated number of malaria deaths for those aged 0–4 years old was calculated using median values of mortality determined by Snow et al. [204]. For the entire period pre-1960 the rate of 9.5/10,000 based on data from six studies. Was used. The population of sub-Saharan Africa was determined annually from 3000 BCE to 2000 CE by linear interpolation from estimates for specific years obtained from the literature [205, 206]. It was assumed that in 10,000 BCE that half the world’s population was living in sub-Saharan Africa, representing 3 million people. Since malaria is largely a rural disease, the rural population for Africa was estimated using data from FAOSTAT (accessed 27th April 2022) between 1950 and 2000. The 1950 estimate for 86% of the population of Africa for earlier years was used in 1950, 42.3% of the population were 0–4 years old compared to 44.3% in 2000 [207]. Annual values between 1950 and 2000 were linearly interpolated. For earlier years, the proportion of 0–4 years old were estimated from a historical study in the Kongo from 1550 to 1750 [203]. In this study the birth rate was 47 births/1000 with a crude mortality rate in children of about 25%. It is assumed that half of these deaths (12.5%) were due to causes other than malaria. Since the total African rural population was 2,580,000 (total population in Africa = 3,000,000 × 0.86 proportion living in rural areas) there would be 121,260 rural births each year with 530,512 births every 5 years (121,260) with 12.5% of these dying from causes other than malaria during this period, resulting in 397,884 survivors, ~ 15.5% of the population (i.e. 464,198/3,000,000). The assumption was that the proportion was constant from year to year before the increase in the population seen in sub-Saharan Africa from 1900 onwards. After 1900, annual values were interpolated linearly using the values given by Tabutin and Schoumaker [208].
It is estimated that 241 million children aged 0–4 years old died from malaria between 3000 BCE and 2000 CE. Making the conservative assumption that 50% of malaria deaths were due to An. gambiae mosquitoes feeding on people suggest that this mosquito was responsible for 120.5 M deaths. In 2021, of the 625,000 malaria deaths that occurred globally, 96% occurred in sub-Saharan Africa [209], living testament to the remarkable ability of this mosquito species, and to An. funestus, to transmit malaria. It should be appreciated that the estimation of deaths from malaria attributed to these mosquitoes is, understandably, an imprecise science with a wide variation in estimates. There are variations between annual population estimates by different authors [205, 210]. Estimating the proportion of children aged 0–4 years old in historical records is based on only one data source and is therefore a biased sample. Importantly, malaria deaths in older age groups or the large number of malaria deaths that would have occurred during pregnancy have been left out of these calculations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Takken, W., Charlwood, D. & Lindsay, S.W. The behaviour of adult Anopheles gambiae, sub-Saharan Africa’s principal malaria vector, and its relevance to malaria control: a review. Malar J 23, 161 (2024). https://doi.org/10.1186/s12936-024-04982-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-024-04982-3